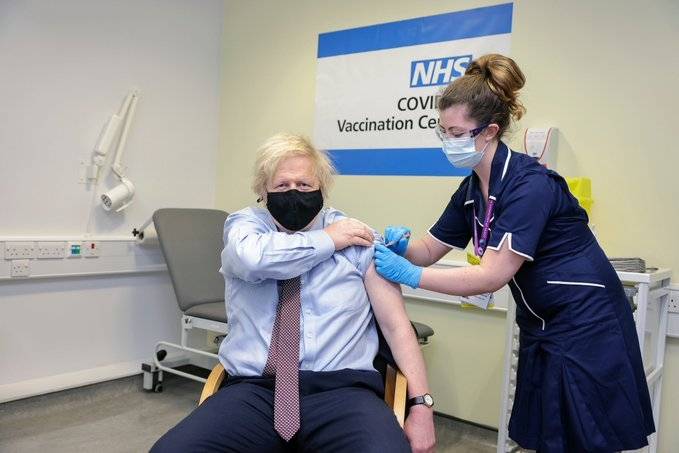
Meanwhile, last week,tThe European Medicines Agency (EMA) has approved the use of the Pfizer-BioNTech Covid-19 vaccine for children aged 12 to 15…reports Asian Lite News.
The European Union’s (EU) new Digital COVID Certificate reached an important milestone on Tuesday when it was launched in the bloc’s seven member states one month ahead of the scheme’s scheduled start on July 1.
It went live in Bulgaria, the Czech Republic, Denmark, Germany, Greece, Croatia and Poland, the European Commission said in a statement, Xinhua news agency reported.

The certificate was proposed by the Commission to enable people to resume safe free travel this summer. The system allows the verification of certificates in a secure and privacy-friendly way.
Available in digital format or on paper, it will provide proof that its holder has been vaccinated against COVID-19, tested negative or recovered from an infection.
The Commission said that the gateway for the certificate had already been tested successfully in 22 countries. The regulation comes into force on July 1 with a phasing-in period of six weeks for the issuance of certificates for those member states that need additional time.
However, those member states that have passed the technical tests and are ready to issue and verify certificates can already start using the system on a voluntary basis.
“The EU Digital COVID Certificate shows the value-added of effective e-health solutions for our citizens,” said Stella Kyriakides, commissioner for health and food safety. “It is important that during the coming weeks, all member states fully finalize their national systems to issue, store and verify certificates, so the system is functioning in time for the holiday season.”
Meanwhile, last week,tThe European Medicines Agency (EMA) has approved the use of the Pfizer-BioNTech Covid-19 vaccine for children aged 12 to 15.
Pfizer-BioNTech becomes the first vaccine to be authorised for adolescents in the 27 member states of the European Union (EU).
Addressing a press conference in Brussels on Friday, Marco Cavaleri, EMA’s vaccine strategy manager, said that the medicines’ regulator had received the necessary data to authorise the vaccine for younger teens.
The data shows that it is highly effective against Covid-19.
He pointed out that the decision needs to be approved by the European Commission and individual national regulators.
Regulators in Canada and the US had already recommended its use for teenagers.
The EMA’s recommendation was based on a study in more than 2,200 adolescents in the US showing that the vaccine was safe and effective.
The trial showed that the immune response in this group was comparable to that in the 16-25 age group.
The study shows that the vaccine was 100 per cent effective at preventing Covid, the EMA said in a statement.
The most common side effects in children aged 12 to 15 are similar to those in people aged 16 and above.
They include pain, tiredness, headache, muscle and joint pain, chills and fever.
ALSO READ-EU-India ties grow amid tensions with China
READ MORE-European nations seek explanation from US on spying row